
- CAREERS |
- CONTACT |
- NEWS |
- CUSTOMER LOGIN






Click here for full press release.
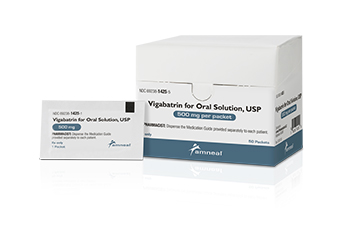
Bridgewater, NJ (USA), May 2, 2018 – Amneal Pharmaceuticals has launched vigabatrin for oral solution USP, a generic equivalent for Sabril®. This is the second generic vigabatrin product currently on the market. Each carton contains fifty 500 mg packets. The finished dosage form is manufactured in the United States.
“We are pleased to add this critical, specialty product to our catalog,” states Amneal EVP-Commercial Operations Andy Boyer. “Bringing a second generic option to this small patient population exemplifies our commitment to delivering products with value to patients and the healthcare system.”
Amneal has also filed an ANDA with US FDA for the tablet form of vigabatrin.
Annual U.S. sales of vigabatrin for oral solution (brand and generic) were $318 million, according to March 2018 IQVIA™ market data.
The Amneal generic product received FDA approval under a Risk Evaluation and Mitigation Strategy (REMS) known as the Vigabatrin REMS program. Information on this program is available at https://www.vigabatrinrems.com/ or 866-244-8175.
Click here to view full prescribing information for vigabatrin.
Click here for a PDF of the complete release.
FOR IMMEDIATE RELEASE
CONTACTS:
Amneal
Apurva Saraf
(631) 742-7674
Impax
Mark Donohue
(215) 558-4526
BRIDGEWATER, NJ, April 27, 2018 – Amneal Pharmaceuticals LLC and Impax Laboratories, Inc. (NASDAQ: IPXL) today announced that the U.S. Federal Trade Commission (FTC) has cleared the Amneal and Impax business combination, subject to Amneal and Impax’s agreement to divest certain products.
The parties have now obtained all regulatory approvals required to close the transaction. Accordingly, the parties expect to consummate the business combination following the close of trading on May 4, 2018. Shares of Impax (IPXL) are expected to cease trading on the NASDAQ stock exchange following the closing of the business combination on May 4, 2018, and the new combined company, Amneal Pharmaceuticals, Inc. (the “Company”), will begin trading on the New York Stock Exchange (NYSE) under the ticker “AMRX” on May 7, 2018. The Company will hold an investor call prior to the start of the trading of Amneal’s Class A common stock on the New York Stock Exchange on May 7, 2018. Pursuant to the business combination agreement, each share of Impax common stock will be converted into the right to receive one share of Amneal Class A common stock.
Under the terms of the consent order with the FTC, Amneal and Impax have agreed to divest a number of marketed and pipeline products to ANI Pharmaceuticals, Inc. (NASDAQ: ANIP), G&W Laboratories, Inc. and Perrigo Company plc (NYSE; TASE: PRGO).
Subject to the consummation of the transaction between Amneal and Impax, ANI will acquire the following Impax products:
• Felbamate Tablets
• Ezetimibe; Simvastatin Tablets
• Desipramine Tablets
• Approved but not commercialized Aspirin; Dipyridamole ER Capsules
• Approved but not commercialized Methylphenidate ER Tablets
• Pending application for Diclofenac; Misoprostol DR Tablets
• Development product Erythromycin Tablets
Subject to the consummation of the transaction between Amneal and Impax, Impax’s interests in the following product will be sold to G&W, who owns the relevant marketing authorization:
• Fluocinonide Topical Cream (emulsified base) 0.05%
Subject to the consummation of the transaction between Amneal and Impax, Impax’s interests in the following products will be sold to Perrigo, who owns the relevant marketing authorizations:
• Azelastine Nasal Spray 0.15%
• Olopatadine Nasal Spray
About Amneal
Amneal Pharmaceuticals LLC, a privately-held company headquartered in Bridgewater, New Jersey, is one of the largest and fastest growing generic pharmaceutical manufacturers in the United States. Founded in 2002, Amneal now has more than 5,000 employees in its operations in North America, Asia, and Europe, working together to bring high-quality, affordable medicines to patients worldwide. Amneal has significantly expanded its portfolio of generic products to include complex dosage forms in a broad range of therapeutic areas.
About Impax
Impax Laboratories, Inc. is a specialty pharmaceutical company applying its formulation expertise and drug delivery technology to the development of controlled-release and specialty generics in addition to the development of central nervous system disorder branded products. Impax markets its generic products through its Impax Generics division and markets its branded products through the Impax Specialty Pharma division. Additionally, where strategically appropriate, Impax develops marketing partnerships to fully leverage its technology platform and pursues partnership opportunities that offer alternative dosage form technologies, such as injectables, nasal sprays, inhalers, patches, creams, and ointments. For more information, please visit Impax’s web site at: www.impaxlabs.com.
Forward-Looking Statements
This communication includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are based on our beliefs and assumptions. These forward-looking statements are identified by terms and phrases such as: anticipate, believe, intend, estimate, expect, continue, should, could, may, plan, project, predict, will, target, potential, forecast, and the negative thereof and similar expressions. Forward-looking statements by their nature address matters that are, to different degrees, uncertain, such as statements about the potential timing or consummation of the proposed transaction or the anticipated benefits thereof, including, without limitation, future financial and operating results. Impax cautions readers that these and other forward-looking statements are not guarantees of future results and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially from those expressed in any forward-looking statements. Important risk factors that could cause actual results to differ materially from those indicated in any forward-looking statement include, but are not limited to: (i) the risk that a condition to effecting the transaction contemplated by the Business Combination Agreement dated as of October 17, 2017, by and among Impax Laboratories, Inc. (“Impax”), Amneal Pharmaceuticals LLC (“Amneal”), Atlas Holdings, Inc. (“Holdco”), and K2 Merger Sub Corporation, as amended by Amendment No. 1, dated as of November 21, 2017, and Amendment No. 2, dated as of December 16, 2017, may not be satisfied; (ii) the ability of Impax and Amneal to integrate their businesses successfully and to achieve anticipated synergies, (iii) the possibility that other anticipated benefits of the proposed transaction will not be realized, including without limitation, anticipated revenues, expenses, earnings and other financial results, and growth and expansion of the new combined company’s operations, and the anticipated tax treatment, (iv) potential litigation relating to the proposed transaction that could be instituted against Impax, Amneal or their respective directors, (v) possible disruptions from the proposed transaction that could harm Impax’s and/or Amneal’s business, including current plans and operations, (vi) the ability of Impax or Amneal to retain, attract and hire key personnel, (vii) potential adverse reactions or changes to relationships with clients, employees, suppliers or other parties resulting from the announcement or completion of the transaction, (viii) potential business uncertainty, including changes to existing business relationships, during the pendency of the business combination that could affect Impax’s or Amneal’s financial performance, (ix) certain restrictions during the pendency of the transaction that may impact Impax’s or Amneal’s ability to pursue certain business opportunities or strategic transactions, (x) continued availability of capital and financing and rating agency actions, (xi) legislative, regulatory and economic developments; (xii) unpredictability and severity of catastrophic events, including, but not limited to, acts of terrorism or outbreak of war or hostilities, as well as management’s response to any of the aforementioned factors; and (xiii) such other factors as are set forth in Impax’s periodic public filings with the Securities and Exchange Commission (the SEC”), including but not limited to those described under the headings “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Information” in Impax’s Form 10-K for the fiscal year ended December 31, 2017, in the Form S-4 filed by Holdco, in the definitive proxy statement on Schedule 14A filed by Impax and in Impax’s other filings made with the SEC from time to time, which are available via the SEC’s website at www.sec.gov. While the list of factors presented here is, and the list of factors to be presented in the proxy statement are, considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward looking statements. Consequences of material differences in results as compared with those anticipated in the forward-looking statements could include, among other things, business disruption, operational problems, financial loss, legal liability to third parties and similar risks, any of which could have a material adverse effect on Impax’s or Amneal’s consolidated financial condition, results of operations, credit rating or liquidity. In light of these risks, uncertainties and assumptions, the events described in the forward-looking statements might not occur or might occur to a different extent or at a different time than Impax has described. All such factors are difficult to predict and beyond our control. All forward-looking statements included in this document are based upon information available to Impax on the date hereof, and unless legally required, Impax disclaims and does not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
# # #
Click here to download the full press release.
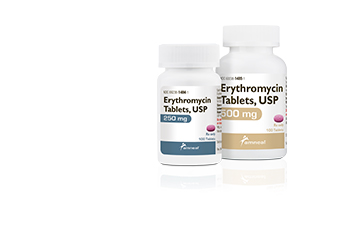
Bridgewater, NJ (USA), March 14, 2018 – Amneal Pharmaceuticals has received FDA approval for Erythromycin Tablets USP, 250 mg and 500 mg strengths. The Amneal product is a therapeutic equivalent for the reference listed drug (RLD) Erythromycin Tablets of Arbor Pharmaceuticals and is the only other immediate release oral tablet available.
“Amneal is committed to increasing access to affordable medications,” said Amneal EVP-Commercial Operations Andy Boyer. “Erythromycin tablets are a great example of a product with limited availability where we can now provide patients and pharmacists with options.”
Amneal’s erythromycin tablets are sold in 100-count bottles and are now available for shipping to wholesalers, distributors and direct to the trade.
Annual U.S. sales of erythromycin tablets were $84 million, according to January 2018 IQVIA™ market data.
Click here to view or download the full press release.
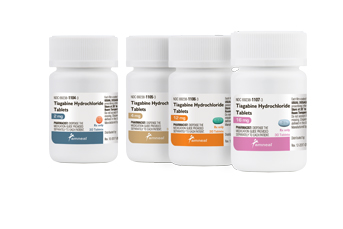
Bridgewater, NJ (USA), March 12, 2018 – Amneal Pharmaceuticals has launched tiagabine hydrochloride tablets in 2 mg, 4 mg, 12 mg and 16 mg strengths. The product is an AB-rated therapeutic equivalent for Gabitril®.
Tiagabine HCl tablets, available in a 30-ct bottles, are now shipping to wholesalers, distributors and direct to the trade.
Annual U.S. sales of Gabitril® and its generic equivalents were $30 million, according to January 2018 IQVIA™ market data.
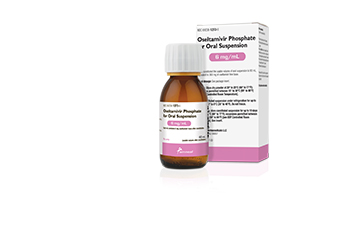
Bridgewater, NJ (USA), March 1, 2018 – Amneal Pharmaceuticals has launched oseltamivir phosphate for oral suspension, the company’s AB-rated therapeutic equivalent for Tamiflu®, in a 6 mg/mL strength. The powder finished dosage form is made in the U.S.A. and packaged in bottles providing 60 mL of usable volume after constitution. Amneal’s oseltamivir phosphate for oral suspension joins its oral solid capsule form approved and launched in July 2017.
“Approval and launch of this liquid form should be of great assistance to patients and caregivers,” states Amneal EVP of Commercial Operations Andy Boyer. “With a severe flu season upon us, our product should be able to help provide relief to thousands of patients. We are pleased to provide this liquid dosage form to patients, particularly the elderly and young who are unable to use an oral solid form.”
Annual U.S. sales of Tamiflu® and its generic equivalents were $956 million, according to December 2017 IQVIA™ market data.
Bridgewater, NJ (USA), February 21, 2018 – Amneal Pharmaceuticals has launched memantine hydrochloride extended-release capsules, the company’s AB-rated equivalent for Namenda XR®, in 7 mg, 14 mg, 21 mg and 28 mg strengths. The product is manufactured here in the United States in the company’s recently expanded Brookhaven, New York facility.
Annual U.S. sales of Namenda XR® were $929 million, according to December 2017 QuintilesIMS market data.
Click here to download full release.
BRIDGEWATER, NJ, February 6, 2018 – Amneal Pharmaceuticals LLC and Impax Laboratories, Inc. (NASDAQ: IPXL), today announced that Andrew S. Boyer, 52, has joined Amneal as Executive Vice President, Commercial Operations and is expected to serve in the same capacity for the new combined company following the consummation of the combination with Impax. Mr. Boyer most recently served as President and CEO of North America Generics, Teva Pharmaceuticals, Inc.
“We are pleased that Andy has joined Amneal and will be leading our commercial organization,” said Chirag Patel, Co-CEO and chairman, Amneal Pharmaceuticals. “Andy is an accomplished executive with more than 20 years of pharmaceutical experience architecting commercial strategies designed to capitalize on changing customer needs and market opportunities. His addition further enhances our current leadership team as well as the proposed leadership team for the new Amneal.”
Mr. Boyer will work closely with Chirag and Chintu Patel, Amneal’s current Co-CEOs, and Rob Stewart, President of Amneal and the future CEO of the new Amneal, to further enhance its business in preparation for the pending combination with Impax, which is currently expected to occur in the first half of 2018.
Mr. Boyer commented, “I am excited to join Amneal at this important time in the company’s evolution and eventual combination with Impax. Amneal has an exceptional generics portfolio and is well regarded by customers for its quality, value and service. I look forward to working with my new colleagues to build upon that strong foundation and lead the combined organization’s continued advancement as a generic pharmaceuticals leader.”
Prior to joining Teva, Mr. Boyer was Senior Vice President of Sales and Marketing for the U.S. Generics Division at Allergan plc (formerly, Actavis plc; formerly, Watson Pharmaceuticals, Inc.) since September of 2006. Mr. Boyer joined Allergan in 1998 as Associate Director of Marketing in Generics. Before joining Allergan, Mr. Boyer served as National Accounts Manager for Lederle/American Cyanamid as well as Marketing Manager for Barr Laboratories. He serves as a Director of Association for Accessible Medicines. Mr. Boyer received his bachelor’s degree in Business Administration and Management from State University of New York at Albany.
About Amneal
Amneal Pharmaceuticals LLC, a privately-held company headquartered in Bridgewater, New Jersey, is one of the largest and fastest growing generic pharmaceutical manufacturers in the United States. Founded in 2002, Amneal now has more than 5,000 employees in its operations in North America, Asia, and Europe, working together to bring high-quality, affordable medicines to patients worldwide. Amneal has significantly expanded its portfolio of generic products to include complex dosage forms in a broad range of therapeutic areas. For more information, visit www.amneal.com.
About Impax
Impax Laboratories, Inc. is a specialty pharmaceutical company applying its formulation expertise and drug delivery technology to the development of controlled-release and specialty generics in addition to the development of central nervous system disorder branded products. Impax markets its generic products through its Impax Generics division and markets its branded products through the Impax Specialty Pharma division. Additionally, where strategically appropriate, Impax develops marketing partnerships to fully leverage its technology platform and pursues partnership opportunities that offer alternative dosage form technologies, such as injectables, nasal sprays, inhalers, patches, creams, and ointments. For more information, please visit Impax’s web site at: www.impaxlabs.com.
Additional Information and Where to Find It
This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval. This communication may be deemed to be solicitation material in respect of the proposed transaction between Impax Laboratories, Inc. (“Impax”) and Amneal Pharmaceuticals LLC (“Amneal”) pursuant to the Business Combination Agreement dated as of October 17, 2017 by and among Impax, Amneal, Atlas Holdings, Inc. (“Holdco”), and K2 Merger Sub Corporation as amended by Amendment No. 1, dated November 21, 2017 and Amendment No. 2 dated December 16, 2017. In connection with the proposed transaction, Holdco filed a registration statement on Form S-4, containing a preliminary proxy statement/prospectus, with the Securities and Exchange Commission (“SEC”) on November 21, 2017. A definitive proxy statement/prospectus will be delivered as required by applicable law after the registration statement on Form S-4 is declare effective by the SEC. This communication is not a substitute for the registration statement, definitive proxy statement/prospectus or any other documents that Impax or Holdco has filed or may file with the SEC or send to stockholders in connection with the proposed business combination. INVESTORS AND SECURITY HOLDERS OF IMPAX ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH THE SEC, INCLUDING THE PROXY STATEMENT/PROSPECTUS, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION.
Investors and security holders will be able to obtain copies of the registration statement, including the proxy statement/prospectus and other documents filed with the SEC (when available) free of charge at the SEC’s website, http://www.sec.gov. Copies of the documents filed with the SEC by Impax or Holdco will be available free of charge on Impax’s internet website at http://www.impaxlabs.com or by contacting Mark Donohue, Investor Relations and Corporate Communications at (215) 558-4526.
Forward-Looking Statements
This communication includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are based on our beliefs and assumptions. These forward-looking statements are identified by terms and phrases such as: anticipate, believe, intend, estimate, expect, continue, should, could, may, plan, project, predict, will, target, potential, forecast, and the negative thereof and similar expressions. Forward-looking statements by their nature address matters that are, to different degrees, uncertain, such as statements about the potential timing or consummation of the proposed transaction or the anticipated benefits thereof, including, without limitation, future financial and operating results. Impax cautions readers that these and other forward-looking statements are not guarantees of future results and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially from those expressed in any forward-looking statements. Important risk factors that could cause actual results to differ materially from those indicated in any forward-looking statement include, but are not limited to: (i) the ability to obtain shareholder and regulatory approvals, or the possibility that they may delay the transaction or that such regulatory approval may result in the imposition of conditions that could cause the parties to abandon the transaction, (ii) the risk that a condition to effecting the transaction may not be satisfied; (iii) the ability of Impax and Amneal to integrate their businesses successfully and to achieve anticipated synergies, (iv) the possibility that other anticipated benefits of the proposed transaction will not be realized, including without limitation, anticipated revenues, expenses, earnings and other financial results, and growth and expansion of the new combined company’s operations, and the anticipated tax treatment, (v) potential litigation relating to the proposed transaction that could be instituted against Impax, Amneal or their respective directors, (vi) possible disruptions from the proposed transaction that could harm Impax’s and/or Amneal’s business, including current plans and operations, (vii) the ability of Impax or Amneal to retain, attract and hire key personnel, (viii) potential adverse reactions or changes to relationships with clients, employees, suppliers or other parties resulting from the announcement or completion of the transaction, (ix) potential business uncertainty, including changes to existing business relationships, during the pendency of the business combination that could affect Impax’s or Amneal’s financial performance, (x) certain restrictions during the pendency of the transaction that may impact Impax’s or Amneal’s ability to pursue certain business opportunities or strategic transactions, (xi) continued availability of capital and financing and rating agency actions, (xii) legislative, regulatory and economic developments; (xiii) unpredictability and severity of catastrophic events, including, but not limited to, acts of terrorism or outbreak of war or hostilities, as well as management’s response to any of the aforementioned factors; and (xiv) such other factors as are set forth in Impax’s periodic public filings with the SEC, including but not limited to those described under the headings “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Information” in Impax’s Form 10-K for the fiscal year ended December 31, 2016, in the Form S-4 filed by Holdco and in Impax’s other filings made with the SEC from time to time, which are available via the SEC’s website at www.sec.gov. While the list of factors presented here is, and the list of factors to be presented in the proxy statement are, considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward looking statements. Consequences of material differences in results as compared with those anticipated in the forward-looking statements could include, among other things, business disruption, operational problems, financial loss, legal liability to third parties and similar risks, any of which could have a material adverse effect on Impax’s or Amneal’s consolidated financial condition, results of operations, credit rating or liquidity. In light of these risks, uncertainties and assumptions, the events described in the forward-looking statements might not occur or might occur to a different extent or at a different time than Impax has described. All such factors are difficult to predict and beyond our control. All forward-looking statements included in this document are based upon information available to Impax on the date hereof, and unless legally required, Impax disclaims and does not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Participants in Solicitation
Impax, Amneal, Holdco and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from Impax’s stockholders in respect of the proposed transaction. Information about the directors and executive officers of Impax is set forth in its proxy statement for its 2017 annual meeting of stockholders, which was filed with the SEC on April 5, 2017, and in its Annual Report on Form 10-K for the year ended Dec. 31, 2016. Other information regarding the participants in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise, is contained in the proxy statement/prospectus regarding the proposed transaction and other relevant materials filed and to be filed with the SEC when they become available. You may obtain free copies of these documents as described in the preceding paragraph. This communication is not intended to and shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote of approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
# # #
Click here to download full release.
Amneal Launches Methylphenidate Hydrochloride Extended-Release Tablets, USP (CII)
AB-Rated Generic for Concerta®
Bridgewater, NJ (USA), February 5, 2018 – Amneal Pharmaceuticals has launched methylphenidate hydrochloride extended-release tablets, USP (CII) in 18, 27, 36, and 54 mg strengths.
Amneal’s methylphenidate is an AB-rated equivalent to the brand Concerta®. The Amneal ANDA included all bioequivalence metrics recommended by FDA.
“We are extremely pleased to add our methylphenidate to the list of therapeutically equivalent generics for Concerta,” commented Amneal EVP – Sales & Marketing Jim Luce. “The entrance of another AB-rated option adds to the choices provided patients, prescribers and payers beyond non-substitutable BX-rated products and the brand.”
Methylphenidate hydrochloride ER tablets are now shipping to wholesalers, distributors and direct to the trade. All strengths are available in 90-ct bottles with a 30-ct size available upon request. The product is manufactured in the U.S.A. at the Amneal plant in Brookhaven, New York.
Annual U.S. sales of methylphenidate ER were $1.5 billion, according to December 2017 IQVIA® market data.
Click here to download full release.
Bridgewater, NJ (USA), February 1, 2018 – Amneal Pharmaceuticals has launched isotretinoin capsules, USP an AB-rated generic for Accutane® in 10 mg, 20 mg, 30 mg and 40 mg strengths. The product is available in cartons containing 3 blister packs of 10 capsules each.
Amneal’s generic received FDA approval under a Risk Evaluation and Mitigation Strategy (REMS) known as the iPledge program. Information on this REMS Program is available at https://ipledgeprogram.com or 866-495-0654.
Click here to view full prescribing information for isotretinoin capsules.
Annual U.S. sales of Isotretinoin capsules were $250 million, according to December 2017 IQVIA™ market data. Isotretinoin capsules began shipping today.
About Amneal
Amneal Pharmaceuticals LLC, a privately-held company headquartered in Bridgewater, New Jersey, is one of the largest and the fastest growing generics pharmaceutical manufacturers in the United States. Founded in 2002, Amneal now has more than 5,000 employees in North America, Asia and Europe, working together to bring high quality affordable medicines to patients worldwide. Amneal has significantly expanded its portfolio of generic products to include complex dosage forms in a broad range of therapeutic areas.
Amneal Pharmaceuticals LLC and Impax Laboratories, Inc. (NASDAQ: IPXL) announced on October 17, 2017 that they have entered into a definitive business combination agreement with the resulting combined company expected to create the 5th largest generics business (by gross revenue) in the United States. The transaction is expected to close in the first half of 2018.
All trademarks listed in this release are property of their respective owners.
###
Download PDF Version of Press Release Here
PSEG Long Island Presents Amneal Pharmaceuticals’ Brookhaven Facility with Energy-Efficiency Rebates Totaling More than $600,000
BRIDGEWATER, NJ, January 23, 2018 – Amneal Pharmaceuticals LLC was today honored by PSEG Long Island for installing energy-efficient systems and processes throughout its flagship Brookhaven, New York, manufacturing facility. At a brief ceremony, PSEG Long Island Director of Energy Efficiency and Renewables Michael Voltz presented Amneal Co-Chairman and Co-CEO Chintu Patel with rebate checks totaling more than $600,000. The Brookhaven site employs more than 600 administrative, manufacturing and R&D employees and manufactures generic oral solids, softgels, high potency and oncological medicines, hormonal medicines, and controlled substances.
“By working together with companies like Amneal Pharmaceuticals, PSEG Long Island is delivering on our promise to be a leader in providing energy efficient solutions for our customers,” said Daniel Eichhorn, president and COO, PSEG Long Island. “Delivering rebates on energy efficient projects helps companies lower their operating costs and save money, which helps make manufacturing on Long Island a viable option.”
The rebates included an LEED (Leadership in Energy and Environmental Design) certification, and a custom rebate based on a whole-building design approach, incorporating a number of energy-efficient measures. The event recognized the completion of all Amneal Brookhaven energy systems installed at its 50 Horseblock Road manufacturing plant, including metal panel walls, LED lighting, motion sensors, high-efficiency chillers, geothermal heating/cooling and a stand-alone sewage treatment plant. PSEG Long Island projects the energy-efficient measures will produce future electricity and gas savings of up to 20 percent.
The improvements made will help the company cut its electric demand by an estimated 462 kilowatts (kW) and reduce its electric consumption by 976,257 kilowatt hours (kWh) per year. This is the equivalent effect of reducing 78.5 homes’ energy use for one year or 156 cars from the road per year.
The project has also received a $1.5 million installment of a scheduled $3 million New York State Consolidated Funding Application grant. It is estimated the project awarded $2 million of work to minority contractors and suppliers.
The installation of the energy systems began in 2013, led by an extended team that included representatives from PSEG Long Island, National Grid, Andrew Desiderio Engineering, JM2 Architecture, the Kulka Construction Corporation, the WSP Corporation, and the Brookhaven Development Corporation.
In addition to the presentation of the rebate checks by PSEG Long Island, Suffolk County Executive Steve Bellone and Town of Brookhaven Supervisor Ed Romaine, offered remarks.
“Amneal is honored to accept this recognition of our efforts to help lead the way for energy-efficient construction on Long Island,” said Chintu Patel. “We thank PSEG Long Island and our many exceptional partners on this project and hope together we demonstrate our belief that practicing energy efficiency is as good for business as it is for protecting our planet.”
About Amneal
Amneal Pharmaceuticals LLC, a privately-held company headquartered in Bridgewater, New Jersey, is one of the largest and the fastest growing generics pharmaceutical manufacturers in the United States. Founded in 2002, Amneal now has more than 5,000 employees in North America, Asia and Europe, working together to bring high-quality affordable medicines to patients worldwide. Amneal has significantly expanded its portfolio of generic products to include complex dosage forms in a broad range of therapeutic areas. For more information, visit www.amneal.com. All trademarks listed in this release are property of their respective owners.
About PSEG Long Island
PSEG Long Island operates the Long Island Power Authority’s transmission and distribution system under a 12-year contract. PSEG Long Island is a subsidiary of Public Service Enterprise Group Incorporated (NYSE:PEG), a publicly traded diversified energy company with annual revenues of $9.1 billion.
Contact:
Cheryl Lechok, Amneal Media Relations
203.613.1506
clechok@optonline.net
Download PDF Version of Press Release Here
Bridgewater, NJ (USA), January 18, 2018 – Amneal Pharmaceuticals has launched budesonide capsules, 3 mg, an AB-rated equivalent to Entocort® EC.
Annual U.S. sales of budesonide and its generic equivalents were $187 million, according to November 2017 IQVIA™ market data. Budesonide capsules are now shipping to wholesalers, distributors and direct to the trade.
Budesonide is the first product launch of the new year for Amneal Pharmaceuticals. In 2017, the company received 45 ANDA approvals (39 final and 6 tentative), and launched 35 product families—nine of these by its injectables subsidiary Amneal Biosciences. For a complete list of Amneal products available in the U.S., visit the online catalog at amneal.com.
About Amneal
Amneal Pharmaceuticals LLC, a privately-held company headquartered in Bridgewater, New Jersey, is one of the largest and the fastest growing generics pharmaceutical manufacturers in the United States. Founded in 2002, Amneal now has more than 5,000 employees in North America, Asia and Europe, working together to bring high-quality affordable medicines to patients worldwide. Amneal has significantly expanded its portfolio of generic products to include complex dosage forms in a broad range of therapeutic areas. For more information, visit www.amneal.com.
All trademarks listed in this release are property of their respective owners.
Photo available upon request.
###
CONTACTS:
Sales:
Jim Luce (sales)
Executive Vice President, Sales & Marketing
M: 949.432.1389
jim.luce@old.amneal.com.php72-34.phx1-1.websitetestlink.com.php7-30.phx1-1.websitetestlink.com
Business Development:
Apurva Saraf
Vice President,
Global Strategy & Corporate Development
Amneal Pharmaceuticals
908.947.3740
apurvas@old.amneal.com.php72-34.phx1-1.websitetestlink.com.php7-30.phx1-1.websitetestlink.com
Media:
Cheryl Lechok
203.613.1506
mediarelations@old.amneal.com.php72-34.phx1-1.websitetestlink.com.php7-30.phx1-1.websitetestlink.com
Download PDF Version of Press Release Here
Bridgewater, NJ (USA), January 4, 2018 – Amneal Biosciences has launched busulfan injection, the company’s AP-rated therapeutic equivalent for Busulfex® 60 mg/10 mL (6 mg/1 mL). The product is supplied in cartons of eight single-dose vials, each containing 60 mg of busulfan in 10 mL of clear sterile solution. It is made without natural rubber latex, gluten or preservatives.
Busulfan injection is now shipping to wholesalers, distributors and direct to the trade.
Annual U.S. sales of Busulfex® were $80 million, according to October 2017 IQVIA™ market data.
About Amneal
Amneal Biosciences, a wholly-owned subsidiary of Amneal Pharmaceuticals LLC, is dedicated to the commercialization of high-barrier-to-entry generic and specialty pharmaceuticals such as injectables, oncologics, anti-infectives and support care for healthcare providers and patients of all ages. The company’s expertise and focus on the unique needs and logistics of this market ensure the same level of quality and service for healthcare institutions and professionals that Amneal delivers to its retail customers.
Amneal Pharmaceuticals LLC, a privately-held company headquartered in Bridgewater, New Jersey, is one of the largest and the fastest growing generics pharmaceutical manufacturers in the United States. Founded in 2002, Amneal now has more than 5,000 employees in North America, Asia and Europe, working together to bring high-quality affordable medicines to patients worldwide. Amneal has significantly expanded its portfolio of generic products to include complex dosage forms in a broad range of therapeutic areas. For more information, visit www.amneal.com.
All trademarks listed in this release are property of their respective owners.
###
CONTACTS:
Sales:
John Niemi
Vice President, Sales
Amneal Biosciences
jniemi@old.amneal.com.php72-34.phx1-1.websitetestlink.com.php7-30.phx1-1.websitetestlink.com
Business Development:
Apurva Saraf
Vice President,
Global Strategy & Corporate Development
Amneal Pharmaceuticals
908.947.3740
apurvas@old.amneal.com.php72-34.phx1-1.websitetestlink.com.php7-30.phx1-1.websitetestlink.com
Media:
Cheryl Lechok
203.961.9280
mediarelations@old.amneal.com.php72-34.phx1-1.websitetestlink.com.php7-30.phx1-1.websitetestlink.com
Download PDF Version of Press Release Here
FOR IMMEDIATE RELEASE
CONTACTS:
Amneal
Apurva Saraf
(908) 947-3740
Impax
Mark Donohue
(215) 558-4526
Amneal Pharmaceuticals and Impax Laboratories to Present at the 36th Annual J.P. Morgan Healthcare Conference
BRIDGEWATER, NJ, January 3, 2018 – Amneal Pharmaceuticals LLC and Impax Laboratories, Inc. (NASDAQ: IPXL) today announced that they will jointly present at the 36th Annual J.P. Morgan Healthcare Conference at the Westin St. Francis in San Francisco on Tuesday, January 9, 2018.
Paul Bisaro, President and CEO of Impax will present the presentation at 8:00 AM Pacific Time (11:00 AM Eastern Time). To access a live webcast of the presentation, visit Impax’s Investor Relations Web site at https://investors.impaxlabs.com/Investor-Relations. The webcast can also be accessed at the following URL: https://jpmorgan.metameetings.net/events/healthcare18/sessions/13440-impax-laboratories-inc/webcast
Following the presentation, Chirag Patel, Co-CEO and Co-Chairman of Amneal, and Bryan Reasons, Senior Vice President and Chief Financial Officer of Impax, will join Mr. Bisaro for a Q&A sessions at 8:30 AM Pacific Time (11:30 AM Eastern Time). To access a live webcast of the Q&A session, visit Impax’s Investor Relations Web site at https://investors.impaxlabs.com/Investor-Relations. The webcast can also be accessed at the following URL: https://jpmorgan.metameetings.net/events/healthcare18/sessions/13846-impax-amneal-pharmaceuticals-q-a/webcast
An archived version will be available approximately one hour after the live presentation and Q&A session ends and can be accessed at the same locations for 90 days.
About Amneal
Amneal Pharmaceuticals LLC, a privately-held company headquartered in Bridgewater, New Jersey, is one of the largest and fastest growing generic pharmaceutical manufacturers in the United States. Founded in 2002, Amneal now has more than 5,000 employees in its operations in North America, Asia,
and Europe, working together to bring high-quality, affordable medicines to patients worldwide. Amneal has significantly expanded its portfolio of generic products to include complex dosage forms in a broad range of therapeutic areas. For more information, visit
www.amneal.com.
About Impax
Impax Laboratories, Inc. is a specialty pharmaceutical company applying its formulation expertise and drug delivery technology to the development of controlled-release and specialty generics in addition to the development of central nervous system disorder branded products. Impax markets its generic products through its Impax Generics division and markets its branded products through the Impax Specialty Pharma division. Additionally, where strategically appropriate, Impax develops marketing partnerships to fully leverage its technology platform and pursues partnership opportunities that offer alternative dosage form technologies, such as injectables, nasal sprays, inhalers, patches, creams, and ointments. For more information, please visit Impax’s web site at: www.impaxlabs.com.
Additional Information and Where to Find It
This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval. This communication may be deemed to be solicitation material in respect of the proposed transaction between Impax Laboratories, Inc. (“Impax”) and Amneal Pharmaceuticals LLC (“Amneal”) pursuant to the Business Combination Agreement dated as of October 17, 2017 by and among Impax, Amneal, Atlas Holdings, Inc. (“Holdco”), and K2 Merger Sub Corporation as amended by Amendment No. 1, dated November 21, 2017 and Amendment No. 2 dated December 16, 2017. In connection with the proposed transaction, Holdco filed a registration statement on Form S-4, containing a preliminary proxy statement/prospectus, with the Securities and Exchange Commission (“SEC”) on November 21, 2017. A definitive proxy statement/prospectus will be delivered as required by applicable law after the registration statement on Form S-4 is declare effective by the SEC.. This communication is not a substitute for the registration statement, definitive proxy statement/prospectus or any other documents that Impax or Holdco has filed or may file with the SEC or send to stockholders in connection with the proposed business combination. INVESTORS AND SECURITY HOLDERS OF IMPAX ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH THE SEC, INCLUDING THE PROXY STATEMENT/PROSPECTUS, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION.
Investors and security holders will be able to obtain copies of the registration statement, including the proxy statement/prospectus and other documents filed with the SEC (when available) free of charge at the SEC’s website, http://www.sec.gov. Copies of the documents filed with the SEC by Impax or Holdco will be available free of charge on Impax’s internet website at http://www.impaxlabs.com or by contacting Mark Donohue, Investor Relations and Corporate Communications at (215) 558-4526.
Forward-Looking Statements
This communication includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are based on our beliefs and assumptions. These forward-looking statements are identified by terms and phrases such as: anticipate, believe, intend, estimate, expect, continue, should, could, may, plan, project, predict, will, target, potential, forecast, and the negative thereof and similar expressions. Forward-looking statements by their nature address matters that are, to different degrees, uncertain, such as statements about the potential timing or consummation of the proposed transaction or the anticipated benefits thereof, including, without limitation, future financial and operating results. Impax cautions readers that these and other forward-looking statements are not guarantees of future results and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially from those expressed in any forward-looking statements. Important risk factors that could cause actual results to differ materially from those indicated in any forward-looking statement include, but are not limited to: (i) the ability to obtain shareholder and regulatory approvals, or the possibility that they may delay the transaction or that such regulatory approval may result in the imposition of conditions that could cause the parties to abandon the transaction, (ii) the risk that a condition to effecting the transaction may not be satisfied; (iii) the ability of Impax and Amneal to integrate their businesses successfully and to achieve anticipated synergies, (iv) the possibility that other anticipated benefits of the proposed transaction will not be realized, including without limitation, anticipated revenues, expenses, earnings and other financial results, and growth and expansion of the new combined company’s operations, and the anticipated tax treatment, (v) potential litigation relating to the proposed transaction that could be instituted against Impax, Amneal or their respective directors, (vi) possible disruptions from the proposed transaction that could harm Impax’s and/or Amneal’s business, including current plans and operations, (vii) the ability of Impax or Amneal to retain, attract and hire key personnel, (viii) potential adverse reactions or changes to relationships with clients, employees, suppliers or other parties resulting from the announcement or completion of the transaction, (ix) potential business uncertainty, including changes to existing business relationships, during the pendency of the business combination that could affect Impax’s or Amneal’s financial performance, (x) certain restrictions during the pendency of the transaction that may impact Impax’s or Amneal’s ability to pursue certain business opportunities or strategic transactions, (xi) continued availability of capital and financing and rating agency actions, (xii) legislative, regulatory and economic developments; (xiii) unpredictability and severity of catastrophic events, including, but not limited to, acts of terrorism or outbreak of war or hostilities, as well as management’s response to any of the aforementioned factors; and (xiv) such other factors as are set forth in Impax’s periodic public filings with the SEC, including but not limited to those described under the headings “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Information” in Impax’s Form 10-K for the fiscal year ended December 31, 2016, in the Form S-4 filed by Holdco and in Impax’s other filings made with the SEC from time to time, which are available via the SEC’s website at www.sec.gov. While the list of factors presented here is, and the list of factors to be presented in the proxy statement are, considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward looking statements. Consequences of material differences in results as compared with those anticipated in the forward-looking statements could include, among other things, business disruption, operational problems, financial loss, legal liability to third parties and similar risks, any of which could have a material adverse effect on Impax’s or Amneal’s consolidated financial condition, results of operations, credit rating or liquidity. In light of these risks, uncertainties and assumptions, the events described in the forward-looking statements might not occur or might occur to a different extent or at a different time than Impax has described. All such factors are difficult to predict and beyond our control. All forward-looking statements included in this document are based upon information available to Impax on the date hereof, and unless legally required, Impax disclaims and does not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Participants in Solicitation
Impax, Amneal, Holdco and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from Impax’s stockholders in respect of the proposed transaction. Information about the directors and executive officers of Impax is set forth in its proxy statement for its 2017 annual meeting of stockholders, which was filed with the SEC on April 5, 2017, and in its Annual Report on Form 10-K for the year ended Dec. 31, 2016. Other information regarding the participants in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise, is contained in the proxy statement/prospectus regarding the proposed transaction and other relevant materials filed and to be filed with the SEC when they become available. You may obtain free copies of these documents as described in the preceding paragraph. This communication is not intended to and shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote of approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
# # #